![]() Cancer Cell
Cancer Cell
A Metabolic-Electrical Remodeling in Cancer
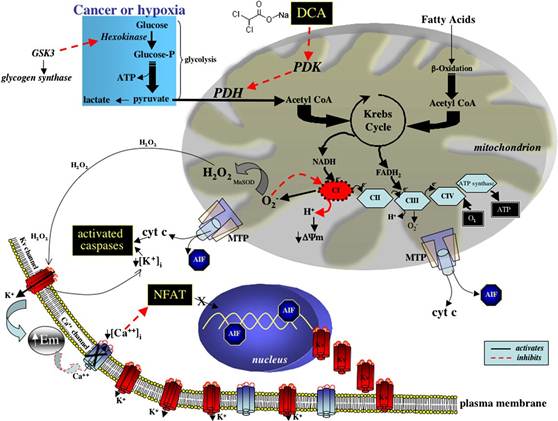
Figure 1. A
Reversible Metabolic-Electrical
Remodeling in Cancer Contributes to Resistance to Apoptosis and Reveals Several
Potential Therapeutic Targets
In cancer, mitochondrial glucose
oxidation is inhibited
and energy production relies on the cytoplasmic glycolysis.
This ‘‘inactivity’’ of
the mito- chondria likely induces a state of apoptosis resistance. Activation of PDH by DCA increases
glucose oxidation by promoting
the influx of acetyl- CoA into the mitochondria and the Krebs cycle,
thus increasing NADH delivery to complex I of the electron transport chain, increasing the production of superoxide, which in the presence of MnSOD is dismutated to the more stable H2O2. Sustained increase in ROS generation can damage the redox-
sensitive complex I, inhibiting H+ efflux and decreasing DJm. Opening of the DJm-sensitive mitochondrial transition pore (MTP) allows the efflux of
cytochrome c and apoptosis inducing
factor (AIF). Both cytochrome c and H2O2 open the redox-sensitive K+ channel Kv1.5 in the plasma membrane
and hyperpolarize the cell (increased Em), inhibiting a voltage-dependent Ca2+ entry. The decreased [Ca2+]i suppresses a tonic activation of NFAT,
resulting in its removal from the nucleus,
thus increasing Kv1.5 expression. The increased efflux of K+ from the cell decreases the tonic inhibition of [K+]i on caspases, further enhancing apoptosis. DCA’s selectivity is based on its ability
to target the unique metabolic profile that characterizes most cancers, and its effectiveness is explained by its dual mechanism of apoptosis induction, both by depolarizing mitochondria (proximal
pathway) and activating/upregulating Kv1.5 (distal pathway).